Fifty shades of Blue: the Maya Blue
We have already discussed about pigments and colours; today we are going to talk about the blue, one of the most appreciated colours in the history of art.
Not surprisingly, in the ancient times the artists had been looking for the most suitable and low-cost materials to use the blue in their artworks. For example, the lapis lazuli was excessively expensive and reserved only for great artworks, whereas the azurite could give stability problems and it was not in the best choice for the frescoes.
Indigo was another blue colourant used in the past. The Indigo has a vegetal origin, as it is extracted from the Indigofera Tinctoria, a shrub of the fabaceae family. This colourant was common in the East since very ancient times and it was especially used in the dyeing of textiles. It was not suitable for the pictorial artworks due to its tendency to easily discolour also in the form of lacquer.
However, in the history of art we meet a pigment based on indigo: the Maya Blue. Mayas’ found the solution to the problem of the use of indigo in painting, creating a brilliant and long-lasting pigment. Maya Blue is incredibly resistant to acid, alkaline and biodegradation attacks.
This is the reason why it is the first organic-inorganic hybrid material synthesized by man known.
Why organic-inorganic?
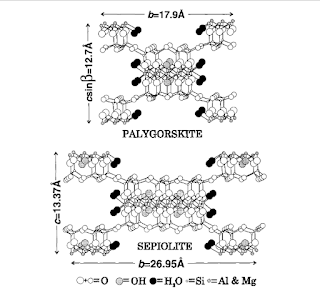 |
| Palygorskite and Sepiolite structures (da E. Galan, Properties and application of Palygorskite-Sepiolite, Clay Minerals (1996) 31,443-453.) |
As alreadymentioned, indigo is a dye of vegetable origin. For the preparation of the Maya Blue, the color was fixed on a matrix based on palygorskite, a phyllosilicate (clay) with the ideal formula Si8(Mg2Al2)O20(OH)2(H2O)4.4H2O: this explains their hybrid nature.
Specifically, palygorskite is a clayey mineral belonging to the sepiolite-palygorskite group; Mineral matter, the structure of these minerals is contained in "sheets" parallel to each other containing zeolites and water molecules. Fe3+ may be present as a replacement for Al3+.
In the preparation of the pigment, the indigo penetrates inside the clay structure, remaining trapped; it is assumed that the lamellar structure of palygorskite is responsible for the stability of the indigo. there are mineralogical differences between sepiolite and palygorskite, whose description would require a separate article; however it is known that the Maya Blue, made with sepiolite, gives a very different result in terms of stability, durability and color compared to the same pigment prepared with palygorskite: therefore it would seem that palygorskite has a fundamental role, thanks to its mineralogical characteristics.
The interaction mechanism between indigo and palygorskite has not been fully elucidated yet. It is assumed that there is the formation of hydrogen bonds between the C=O and N-H units of clay and indigo, or the formation of hydrogen bonds between the indigo carbonyls and the water of the clay structure. It is also possible that there is a direct link between the octahedral cations of Mg2+ and Al3+ and the indigo molecules without water.
Unfortunately, there are no written documents testifying the preparation of the Maya Blue: just think that this pigment was officially discovered only in 1931 by Mervin.
Two main methods of preparation have been proposed in the literature: the dry crushing of indigo and palygorskite or the tincture in the tank using an aqueous suspension of the clay. In the first case, it has been shown that most of the dye can not penetrate the clay structure (only 1-2% by weight is successful), making it necessary to be heated between 130° and 180° (Cabrera-Garrido), 1969; Torres, 1988; Reyes-Valerio, 1993; Sánchez del Río et al., 2006). Depending on the heating temperature, the hue is affected.
However, although there is no unequivocal agreement about color formation, its stability in clay and the very mode of pigment preparation, it is generally accepted that the main responsible for the color of Maya Blue is the bathochromic shift of the absorption of the pigments.
If you are interested in knowing more about the scientific debate about the Maya Blue, here are some references to help you satisfy the curiosity!
Francesca
• Doménech-Carbò, S. Holmwood, F. Di Turo, N. Montoya, F. Manuel Valle-Algarra, H.G.M. Edwards, and M. T. Doménech-Carbó, On the Composition and Color of Maya Blue: A Reexamination of Literature Data Based on the Dehydroindigo Model, The Journal of Physical Chemistry C, 2019, In press.
• E. Galan, Properties and application of Palygorskite-Sepiolite, Clay Minerals (1996) 31,443-453.
• Doménech-Carbò, M.T. Doménech-Carbò, C. Vidal-Lorenzo, M.L. Vazquez de Agredos-Pascual, L. Osete-Cortina, F. M. Valle-Algarra, Discovering of indigoid-containing clay pellets from La Blanca: significance with regard to the preparation and use of Maya Blue, Journal of Archaeological Science 41 (2014) 147-155.
• Doménech-Carbò, M.T. Doménech-Carbò, F. M. Valle-Algarra, M. E. Domine, L. Osete Cortina, On the dehydroindigo contribution to Maya Blue, Journal of Material Science (2013), 48:7171-7183.
• D. Reinen, P. Köhl, and C. Müller Marburg, The Nature of the Colour Centres in ‘Maya Blue’ - the Incorporation of Organic Pigment Molecules into the Palygorskite Lattice, Zeitschrift fur Anorganische und Allgemeine Chemie.
• Doménech-Carbó, M. T. Doménech-Carbó, L. Osete-Cortina, F. M. Valle-Algarra, D. Buti, Isomerization and redox tuning in ‘Maya yellow’ hybrids from flavonoid dyes plus palygorskite and kaolinite clays, Microporous and Mesoporous Materials 194 (2014) 135–145.
• E. Sanz, A. Arteaga, M.A. García, C. Cámara, C. Dietz, Chromatographic analysis of indigo from Maya Blue by LC-DAD-QTOF, Journal of Archaeological Science 39 (2012) 3516-3523.
• Merwin, H.E., 1931. Chemical analysis of pigments. In: Morris, E.H., Charlot, J., Morris, A.A. (Eds.), The Temple Warriors at Chitchen Itzá, Yucatán, Carnegie Institution of Washington Publication 406. Carnegie Institution of Washington, Washington, pp. 355-356.).


Comments
Post a Comment